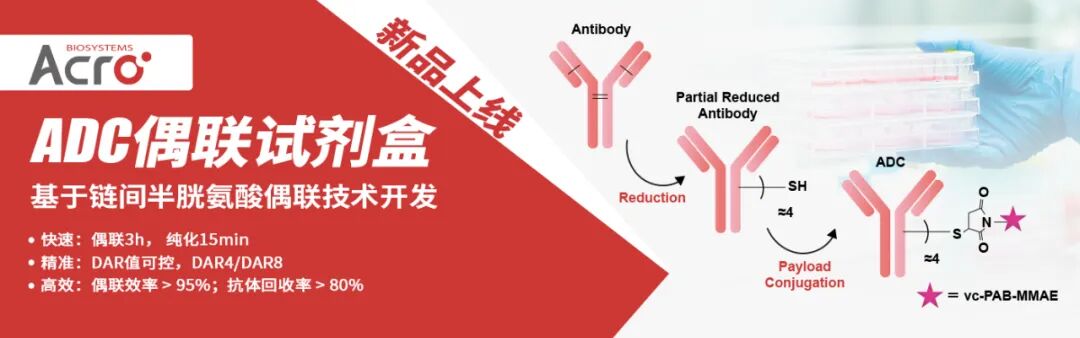
ADC(抗体药物偶联物)是 “抗体 - 连接子 - 细胞毒性载荷” 构成的靶向药物,核心是通过偶联技术将细胞毒性载荷(如MMAE、DXd)精准连接到抗体上,实现“靶向递送毒性药物至肿瘤细胞” 的效果 ——偶联技术决定载荷如何高效、稳定地与抗体结合,直接影响 ADC 的药效、安全性和生产可行性。
-
偶联流程漫长,动辄耗费数天甚至更久,严重拖慢研发节奏?
-
DAR值像“开盲盒”,难以精确控制,导致产物异质性高,影响药效评估?
-
抗体活性和回收率在偶联过程中损耗严重,宝贵样品令人心疼?
ACROBiosystems百普赛斯全新推出的抗体偶联试剂盒,为您带来“快、准、稳、简” 的ADC制备体验,保证抗体活性与高回收率,得到的ADC产物可用于细胞或动物实验,以筛选合适的候选ADC药物。
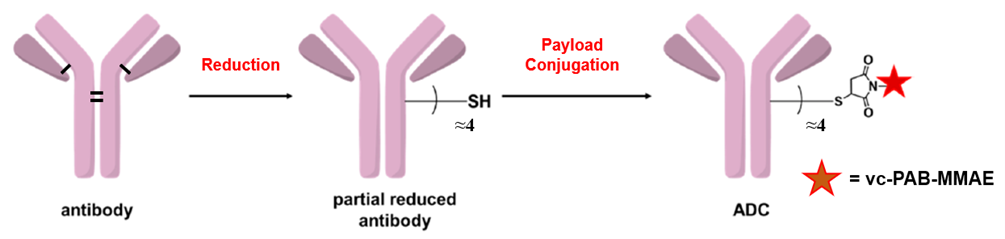
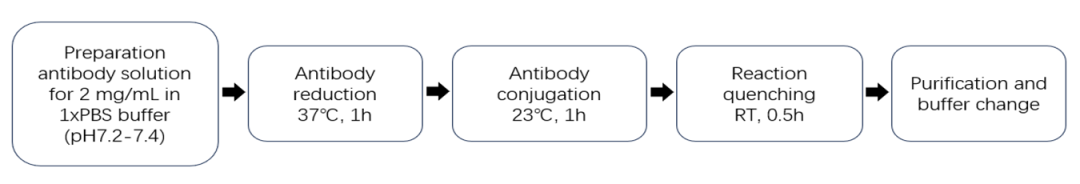
本试剂盒基于链间半胱氨酸偶联技术开发:抗体的链间二硫键可以被还原产生内源性链间半胱氨酸残基,具有马来酰亚胺官能团的有效载荷药物可以特异性偶联这些半胱氨酸残基并形成结构稳定的ADC。依靠这种经典的偶联策略,本试剂盒能够实现快速可控的ADC制备,只需要3个小时即可获得平均药物抗比率(DAR)为4.0±0.5的ADC偶联物,且获得的ADC偶联物表现出显著的细胞毒性作用,为ADC药物筛选和机制研究提供了可靠的实验工具。
-
偶联时间短:3 小时内即可生成抗体药物偶联物(ADCs)
-
药物抗体比率(DAR)值可控:可精准调控 DAR,同时保留良好抗体活性
-
偶联效率与回收率高:偶联效率 > 95%,抗体回收率 > 80%
-
无需高浓度抗体:仅需 2 mg/mL 的抗体浓度即可实现反应
-
纯化简单:使用离心脱盐柱,15 分钟内即可完成纯化
|
货号 |
产品名称 |
|
ADC Conjugation Kit (MMAE, DAR4, 200ug, for human IgG1) |
|
|
ADC Conjugation Kit (MMAE, DAR4, 1mg, for human IgG1) |
|
|
ADC Conjugation Kit (MMAF, DAR4, 200ug, for human IgG1) |
|
|
ADC Conjugation Kit (MMAF, DAR4, 1mg, for human IgG1) |
|
|
ADC Conjugation Kit (Exatecan, DAR8, 200ug, for human IgG1) |
|
|
ADC Conjugation Kit (Exatecan, DAR8, 1mg, for human IgG1) |
试剂盒组分(以MMAE, DAR4, 货号:ADC-P013为例):

以下ADC产品使用ADC偶联试剂盒(MMAE, DAR4, 货号:ADC-P013)制备。
抗体信息:曲妥珠单抗生物类似物。
✅DAR值及纯度经HPLC验证 (HIC+SEC):
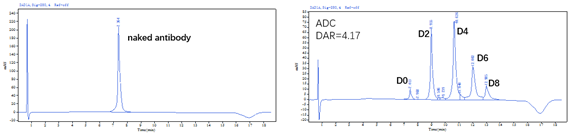
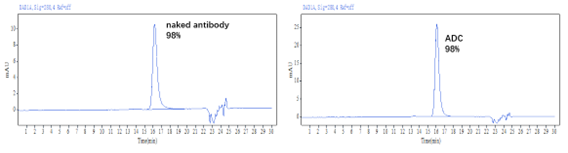
Figure 1. The ADC was prepared using the ADC Conjugation Kit (MMAE, DAR4) and analyzed by HIC and SEC-HPLC. The average drug-antibody ratio (DAR) is 4.0±0.5, and the purity of the ADC is greater than 95%.
✅DAR值经质谱验证
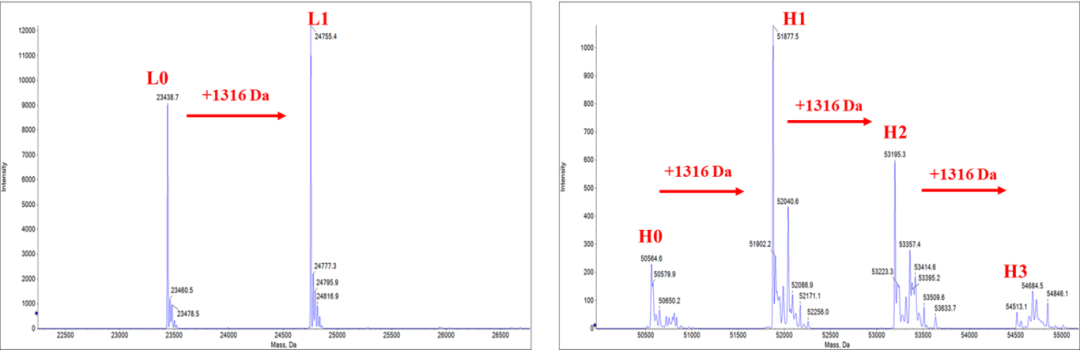
Figure 2. The DAR (3.82) was calculated from the weighted average of the deconvoluted MS peak areas using LC-MS/MS. The results showed the deconvoluted mass spectra of light chains and heavy chains, and the increase in molecular weight caused by the coupling payload (1316±3 Da). The heterogeneity in N-glycosylation of heavy chain adds to the complexity of the mass spectrum.
✅抗原结合能力检测
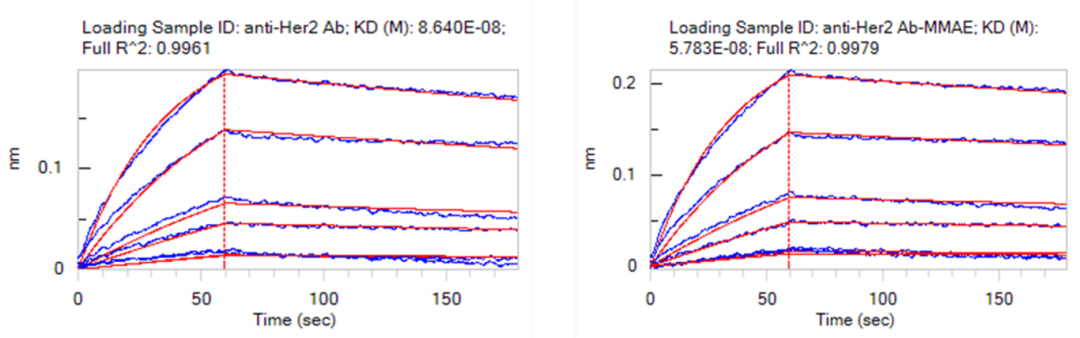
Figure 3. Binding affinity of anti-Her2 antibody and anti-Her2 antibody–MMAE conjugate to human Her2 (Cat. No. HE2-H5225) as determined by BLI (Bio-Layer Interferometry). The conjugate exhibits nanomolar affinity (0.57 nM) for human Her2, comparable to the naked antibody (0.86 nM).
✅体外细胞毒性验证
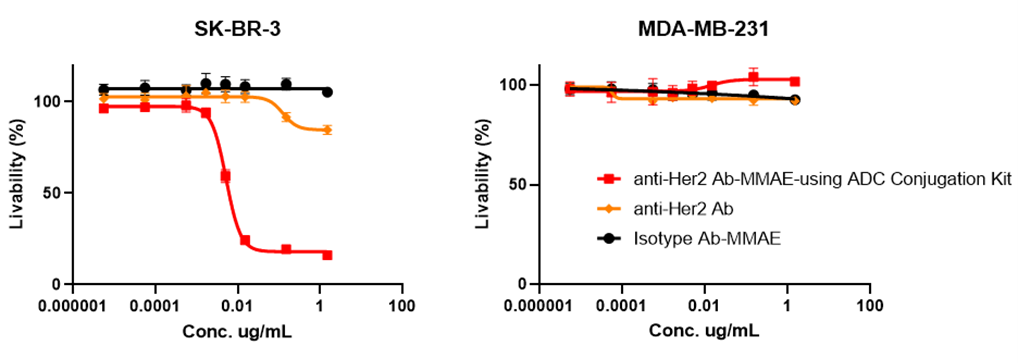
Figure 4. In vitro cytotoxicity assays: The ADC can bind and internalize in target cells (SK-BR-3) with high expression of HER2 and release MMAE inside the cells to induce a cytotoxic effect (IC50=0.0058 µg/mL). Meanwhile, no cytotoxicity was observed in HER2 receptor-nagetive cell lines (MDA-MB-231).
✅不同规格Kit一致性验证

Figure 5. In vitro cytotoxicity of products prepared by different size of the ADC Conjugation Kit (Cat. No. ADC-P013, ADC-P014). The result shows very high consistency (RSD<10%).
✅稳定性验证
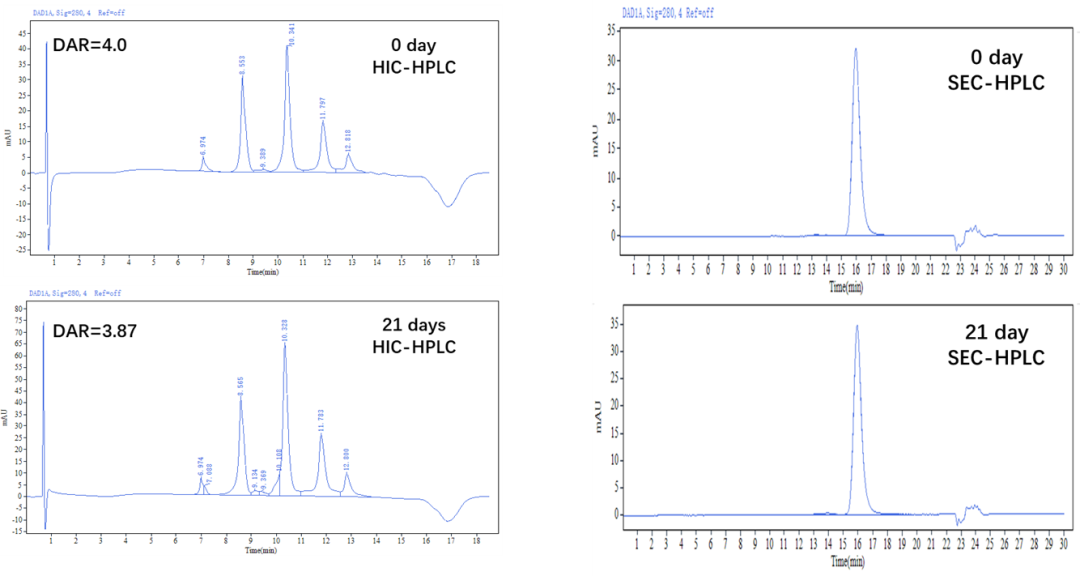
Figure 6. The MMAE-ADC was prepared using the ADC Conjugation Kit, which were left at 37℃ for 21 days. The ADCs were analyzed by HIC and SEC-HPLC. The average drug-antibody ratio (DAR) is 4.0±0.5, and the purity of the ADC is greater than 95%.

除此之外,ACROBiosystems百普赛斯推出了AGLink®新型DAR2&4定点偶联试剂盒,该试剂盒基于YTConju™糖基偶联技术平台开发,包含毒素(MMAE)、反应官能团(Tz/DBCO)、检测标签(Biotin)三大模块,为偶联药物的早期研究及相关生物学实验提供强大支持。


ACROBiosystems
inquiry@acrobiosystems.com
15117918562
(备注:姓名+公司)